Autophagy
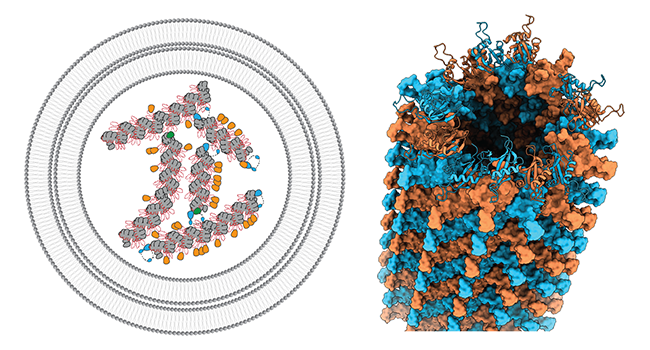
p62/SQSTM1 can be considered one of the archetypical autophagy receptors, which is centrally involved in the recognition and subsequent disposal of ubiquitinated cargo through autophagy. Moreover, p62 is also known to act in a wide range of signaling activities affecting nutrient sensing, oxidative stress, infection, immunity and inflammation. We have shown that p62 forms flexible filamentous structures by an N-terminal PB1 domain-scaffold that hosts a C-terminal binding platform with critical interaction motifs (Ciuffa et al. 2015). In the cellular environment, p62 filaments have been localized in so-called p62 bodies (Jakobi et al., 2020) that were shown to display properties of liquid-liquid phase separation. We study the structural biology of these filamentous biomolecular condensates and will further shed light on their structural and functional interaction partners in the cell (recently reviewed in Berkamp, Mostafavi et al. 2020).
Related Publications
- Ciuffa, R., Lamark, T., Tarafder, A.K., Guesdon, A., Rybina, S., Hagen, W.J.H., Johansen, T., and Sachse, C. The Selective Autophagy Receptor p62 Forms a Flexible Filamentous Helical Scaffold. (2015) Cell Rep 11, 748–758.
- Jakobi A.J., Huber S.T., Mortensen S.A., Schultz S.W., Palara A., Kuhm T., Shrestha B.K., Lamark T., Hagen W.J.H., Wilmanns M., Johansen T., Brech A., and Sachse C. (2020) Structural basis of p62/SQSTM1 helical filaments and their role in cellular cargo uptake. Nat Commun 11(1):440.
- Berkamp S., Mostafavi S., and Sachse C. (2020) Structure and Function of p62/SQSTM1 in the emerging framework of phase separation. FEBS Letters doi: 10.1111/febs.15672.
ESCRT machinery
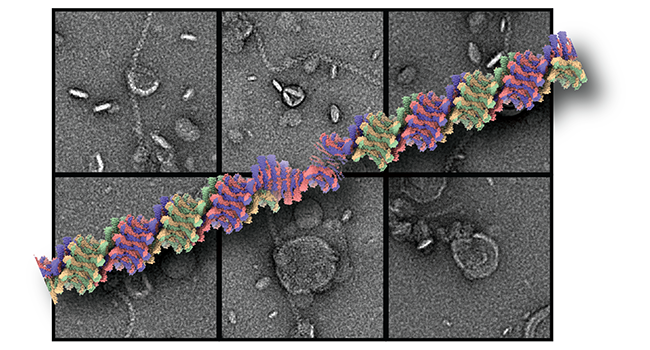
ESCRT machinery proteins mediate a range of cellular membrane remodeling activities that were initially described in multivesicular biogenesis and henceforth received their designation endosomal sorting complexes for transport (ESCRT). Central to these processes is the assembly of ESCRT-III subunits into polymeric structures. There are multiple isoforms that a known to hetero-complex structures performing the central membrane remodeling steps. Recently, we determined the cryo-EM structure of a helical assembly of S. cerevisiae Vps24 at 3.2 Å resolution and found that Vps24 adopts an elongated open conformation (Huber et al., 2020). We study the interaction with lipid membranes together with other ESCRT-III members such as Vps24, Vps2 and Snf7. In vitro, liposomes are deformed into neck and tubular structures by an ESCRT-III heteropolymer coat. We aim to understand the assembly structure and the mode of membrane interactions. The results will finally reveal their contribution to membrane scission events in different cellular circumstances such as cytokinesis, viral budding, nuclear envelope sealing and many more.
Related Publications
- Huber S.T., Mostafavi S., Mortensen S.A., Sachse C. Structure and assembly of ESCRT-III helical Vps24 filaments. (2020) Sci Advances 6(34): eaba4897.
- Junglas B., Huber S.T., Heidler T., Schlösser R., Mann D., Hennig R., Clarke M., Hellmann N., Schneider D. and Sachse C. PspA adopts an ESCRT-III-like fold and remodels bacterial membranes. (2021) Cell online 23rd of June.
